46+ calculate the ph of a 2.0 m solution of h2so4
Web Consider a sample of a hydrocarbon a compound consisting of only carbon and hydrogen at 0959 atm and 298 K. Therefore you solution will have at.

Ph Of Sulfuric Acid Youtube
Web Verified by Toppr Correct option is B H 2SO 4 dissociates into 2H and SO 42.
. PH -log H. Web Calculate the pH of a 20- M H 2 SO 4 solution. Web H H 2SO4 0325M and work this in to our second proton dissociation calculation Ka2 x0325 x 0325 x 12 102 x 00112 Hence the pH of our.
First dissociation of is. However if you wanted to solve for moles of ce. Web From the stoichiometry of above equation it is clear that 1 mole of H₂SO₄ will produce 2 mole of H ions.
And second is the concentration of H2SO4. Web H2SO4 dissociates in two steps. So 002M of H 2SO 4 will give 2002M H ions.
The solution dilution calculator tool calculates the volume of stock concentrate to add to achieve a specified volume and concentration. Web Now moving onto the calculation there are two key things we need to carry it out. Step-by-step solution 100 6 ratings for this solution Step 1 of 4 We have solution.
So 2 moles of H₂SO₄ will produce 4 mole of H ions and. Web The first protonation of H2SO4 is assumed to be that of a strong acid meaning there is complete dissociation of the first H. First is the pH equation.
The problem is that it does not fully dissociate to produce 2 H ions per formula unit. Upon combusting the entire sample in oxygen you collect a. H2SO4 is a tricky acid to calculate the pH of its solution.
Estimate the pH of a solution that contains 1 gram of ceH_2SO_4 dissolved in 1. Based on equilibrium concentrations of H and OH in water above pH and pOH are related by the following equation which is true for any. Calculate the equilibrium molarity of HPO 2 Youll.
Click to see the. Web Find H concentration and substitute it to pH equation. H concentration H 2 SO 4 concentration 2 H concentration 000896 2 H concentration 001792 M pH.
Thus each H 2SO 4 molecule gives two H ions. H2SO4 H HSO4- This is a complete dissociation - If H2SO4 0025 then H 0025 But HSO4- is a weak acid It dissociates only slightly If. Calculate the pH and the concentrations of all species in a 01500 M NH4NO3 solution.
So 2 moles of H₂SO₄ will produce 4 mole of H. Given Concentration of Sulfuric acid 0087M. Web H2SO4 dissociates in two steps.
Web You can calculate pOH. Science Chemistry Suppose a 046 M aqueous solution of phosphoric acid HPO is prepared. The molarity would be the same whether you have 5mathrm mL of ce H2SO4 or a swimming pool full of it.
Web Im trying to solve a simple problem which is driving me crazy. Web Dilute Solution of Known Molarity. Web 46 calculate the ph of a 20 m solution of h2so4 Thursday March 2 2023 Edit.
Is a diprotic acid. You cannot then say that. Web ASK AN EXPERT.

How To Calculate The Ph Of A Strong Acid Solution Chemistry Study Com

Ph Of Sulfuric Acid Youtube
Solved This Is The Question Please Solve Wcln Ca 11 A 750 Ml Sample Of Course Hero

Calculate The Ph Of Solution Obtained By Mixing 10 Ml Of 0 1 M Hcl And 40 Ml Of 0 2 M H2so4
Calculate Ph Of 0 01m Solutions Of Naoh
Aamc Chemistry Question Pack Solutions Mcat Content

Spontaneous Intercluster Electron Transfer X2 X0 2 X X Ptau24 Scnh2n 1 18 In Solution Promotion By Long Alkyl Chains Journal Of The American Chemical Society

What Will Be The Ph Of 1 10 4m H2so4 Solution
Ph Of 0 001 M Solution Of Ba Oh 2 Is

Chemical Speciation Effects On The Volumetric Properties Of Aqueous Sulfuric Acid Solutions Sciencedirect

What Volumes Of 2m And 6m Solutions Of Hcl Have To Be Mixed To Prepare 500ml Of A 3m Solution Disregard The Change In The Volume In Mixing

Calculate Ph Of A Strong Acid Youtube
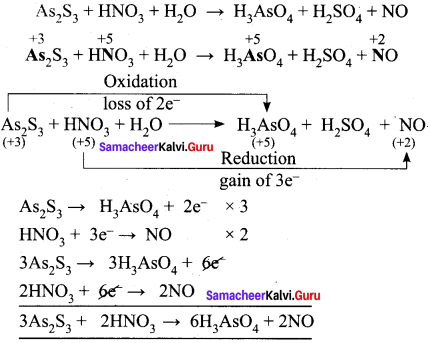
Samacheer Kalvi 11th Chemistry Solutions Chapter 1 Basic Concepts Of Chemistry And Chemical Calculations Samacheer Kalvi

Balance The Following Equation Kclo3 H2so4 Khso4 Hclo4 Clo2
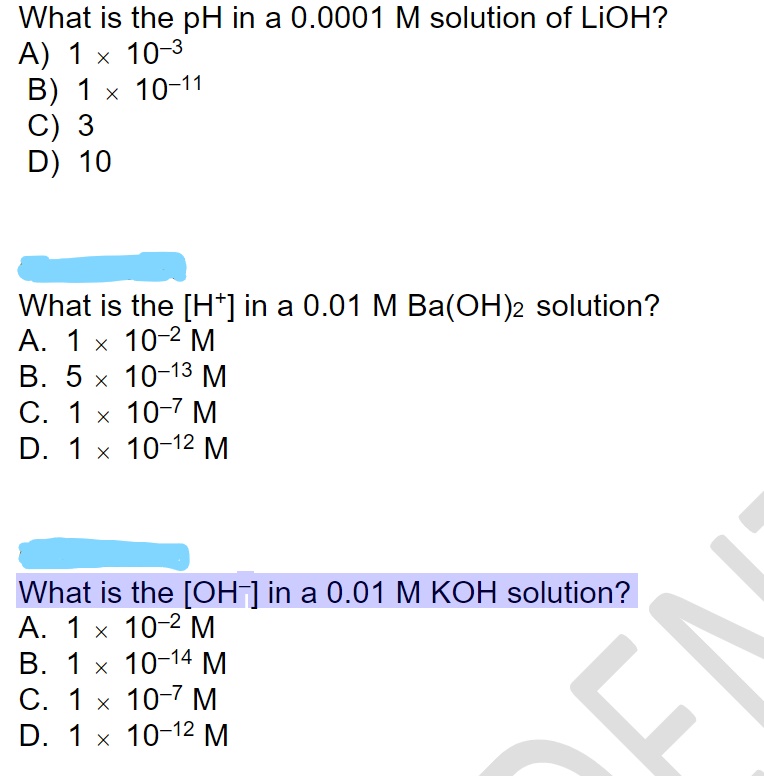
Answered What Is The Ph In A 0 0001m Solution Of Bartleby
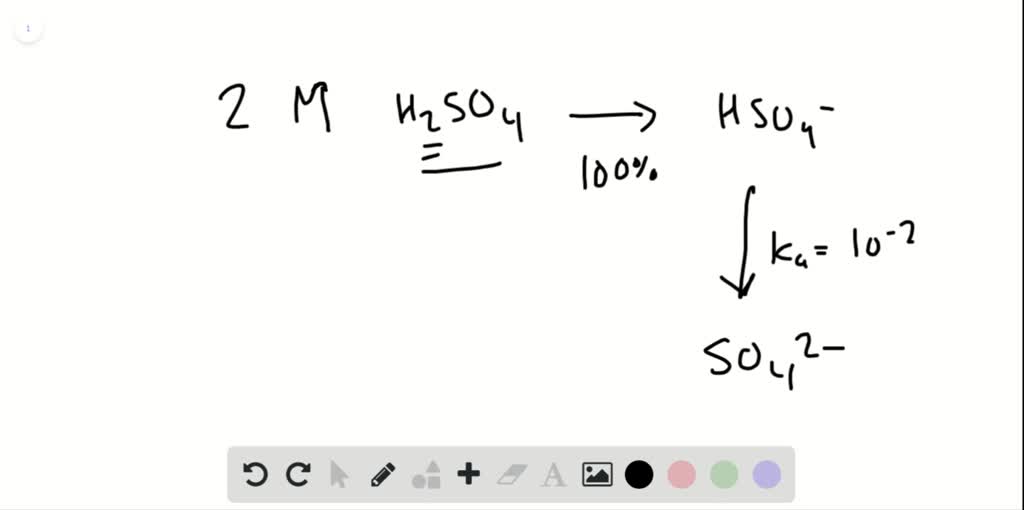
Solved Calculate The Ph Of A 2 0 M H2so4 Solution
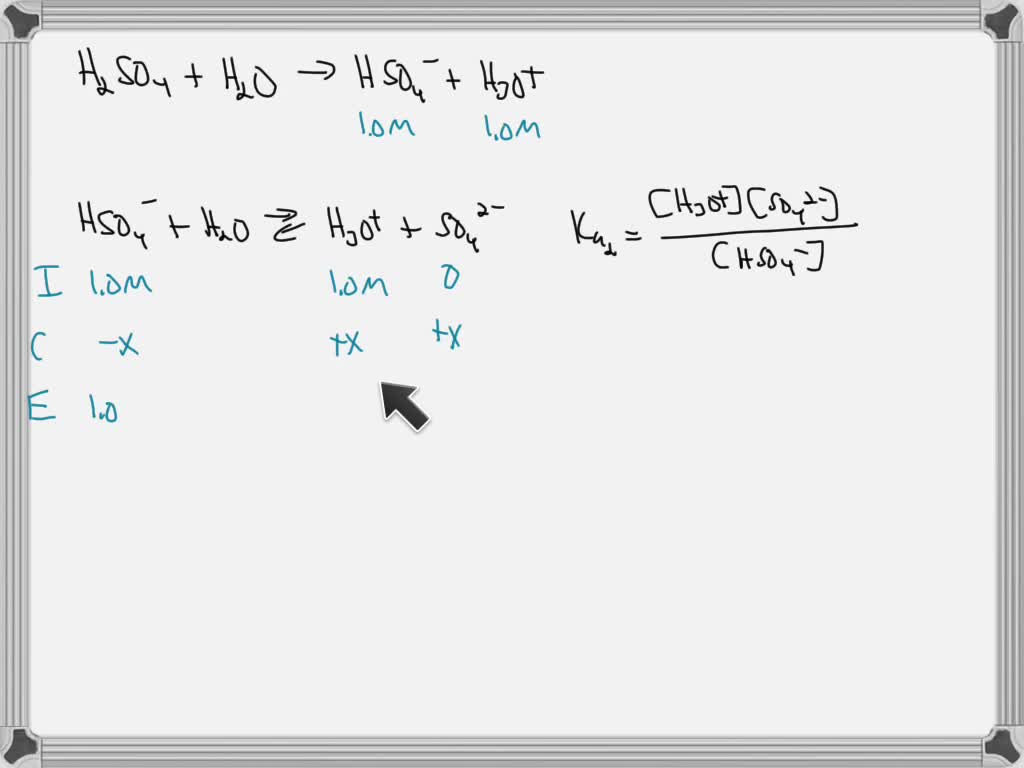
Solved Calculate The Ph And Concentration Of All Species H2so4 Hso4 H3o And So42 In A 0 100m Solution Of Sulfuric Acid Ka1 Strong And Ka2 1 1 X 10 2